In the wake of rising concerns over the bird flu, attention has turned sharply towards the biotech sector, particularly companies like Moderna, known for their pioneering mRNA vaccines. As the world grapples with the outbreak of avian flu, the stock market has seen notable movements, with Moderna stock alongside Pfizer stock experiencing surges. This phenomenon underscores the crucial role of vaccine production in public health, highlighting the importance of flu vaccine research and mRNA vaccines in combating pandemic threats. The intersection of public health crises and market dynamics presents a potent reminder of how vital vaccine development, from vaccine trials to vaccine production, is to global safety and economies.
This article will delve into the bird flu outbreak as a critical catalyst in the vaccine stock rally, focusing on Moderna (nasdaq: mrna) and its performance amidst growing public health concerns. We will analyze the market reactions to the surge in vaccine-related stocks, provide technical analysis of affected stocks including Moderna and Pfizer, and discuss global responses to vaccine production and future prospects for mRNA vaccine programs. Furthermore, we will explore the implications of the flu season on vaccine news and the wider landscape of vaccine contracts and trials, offering insights into the Moderna stock forecast and its significance for investors. Through a comprehensive overview, we aim to inform readers about the intertwining realms of vaccine development, public health, and financial markets, emphasizing the impact of ongoing health crises on moving stocks and the broader implications for society.
The Bird Flu Outbreak: A Catalyst for Vaccine Stocks
Recent Surge in Bird Flu Cases
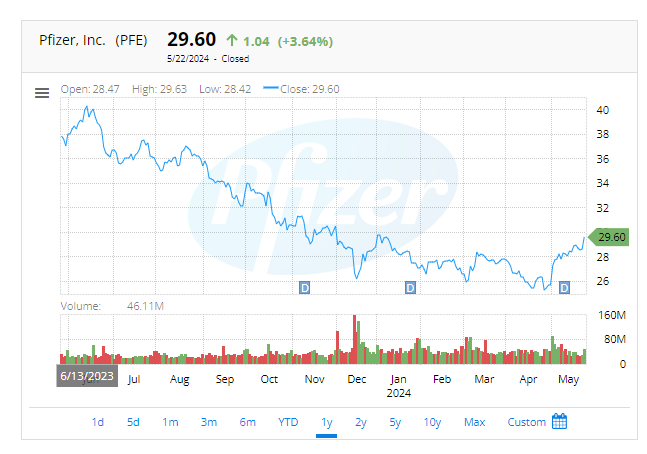
The bird flu outbreak has triggered a significant response in the financial markets, particularly among vaccine stocks. Following Australia’s first human case of avian influenza, shares of Moderna (NASDAQ:MRNA) and other vaccine developers saw substantial gains. Moderna’s stock surged by 13.7%, while Pfizer (NYSE:PFE) experienced a 3.6% increase. This uptick in stock prices reflects the growing concerns over the bird flu’s potential impact on public health and the economy.
Moderna and Pfizer in Talks with the U.S. Government
In response to the escalating bird flu cases, Moderna and Pfizer are actively engaging in discussions with the U.S. government regarding the development of vaccines for bird flu. These talks underscore the critical role that mRNA vaccines, known for their rapid development capabilities, may play in addressing the outbreak. Analysts at UBS have highlighted mRNA vaccines’ unprecedented speed and capabilities, noting the current uncertainty around the virus’s potential for human transmission and the broader public health risk.
Impact on Moderna, BioNTech, and Pfizer Stock Prices
The bird flu outbreak has had a notable impact on the stock prices of key players in the vaccine development sector. CureVac N.V. (CVAC) shares experienced a 25% increase, while Moderna Inc. (MRNA) and BioNTech SE’s (BNTX) stocks gained 14% and more than 10%, respectively. This surge in stock prices can be attributed to the mounting cases of bird flu in humans, which has attracted the attention of day traders and investors alike. The increased focus on vaccine developers like Moderna, Pfizer, and BioNTech reflects the market’s anticipation of their crucial role in combating the outbreak.
This section outlines the direct correlation between the bird flu outbreak and the rallying of vaccine stocks, highlighting the ongoing discussions between vaccine developers and the U.S. government, and the resultant impact on stock prices.
Market Reactions: Analyzing the Surge in Vaccine-Related Stocks
Historical context of outbreaks boosting vaccine stocks
Outbreaks of infectious diseases have historically led to surges in the stock prices of vaccine manufacturers. This pattern was observed during the H1N1 swine flu pandemic in 2009 and the Ebola outbreak in 2014-2016, where vaccine-related companies experienced significant gains in their stock values.
Day traders and retail investors’ role
The recent surge in vaccine-related stocks has also been driven by increased interest from day traders and retail investors. These groups have been actively following the progress of vaccine development, especially for COVID-19, and have capitalized on the potential upside of companies involved in vaccine production.
Analysts’ perspectives on stock movements
Analysts have provided insights into the factors driving changes in the stock prices of vaccine-related companies. While some caution that current stock prices may be inflated, others highlight the long-term potential of these companies, given the expected sustained global demand for vaccines.
| Factor | Impact on Vaccine-Related Stocks |
|---|---|
| Historical Outbreaks | Positive surge in stock prices |
| Day Traders & Retail Investors | Increased trading activity and interest |
| Analysts’ Insights | Mixed perspectives on stock valuation |
The table summarizes the key factors influencing the market reactions to vaccine-related stocks. These include the historical context of outbreaks boosting stock prices, the role of day traders and retail investors in driving up stock prices, and the varied perspectives of analysts on the sustainability and valuation of these stocks.
Technical Analysis of Affected Stocks
Moderna Inc. (MRNA)
Moderna’s stock performance has been notably robust, reflecting investor confidence and market response to its ongoing developments in vaccine technology. On a specific trading day, Moderna’s stock saw an impressive surge, closing up approximately 14%, marking its biggest one-day gain since 2022. This spike was largely attributed to the company’s announcement about a vaccine in phase 2 development that targets an avian influenza virus, which is related to the current outbreak. The technical indicators reveal a strong buy signal, with a high volume of trades pushing the stock to new 52-week highs. The Relative Strength Index (RSI) and Moving Average Convergence Divergence (MACD) levels indicate strong buying momentum, suggesting continued investor interest.
BioNTech SE (BNTX)
Despite not having a bird flu vaccine currently in development, BioNTech’s experience with mRNA technology positions it advantageously to potentially expedite vaccine production if needed. The company’s stock also experienced a significant rise, closing up around 11%. This increase reflects the market’s anticipation of BioNTech’s capability to leverage its technological expertise swiftly in response to public health needs. The stock’s technical analysis shows a predominantly neutral outlook with a few indicators suggesting buy positions, highlighting cautious optimism among investors.
Comparative Performance Over the Past Week
Over the past week, both Moderna and BioNTech have demonstrated significant stock price movements. Moderna’s stock increased by 13.67%, while BioNTech’s stock rose by 12.19%. These movements underscore the reactive nature of biotech stocks to global health concerns and the potential for rapid development of necessary vaccines. The comparative performance of these stocks highlights the market’s confidence in their respective vaccine development capabilities, especially in light of the ongoing public health challenges.
Global Response and Future Prospects for Vaccine Production
Government and Health Organizations’ Involvement
Government and health organizations have been pivotal in addressing the COVID-19 pandemic, which has set a precedent for their role in the global response to other infectious diseases such as avian flu. Their involvement is crucial not only in facilitating vaccine production but also in coordinating international efforts to manage outbreaks effectively.
mRNA Technology in Combating Avian Flu
The development of mRNA technology has revolutionized the approach to vaccine production, offering promise against various infectious diseases, including avian flu. This technology enables rapid development and deployment of vaccines, which is vital in responding to fast-spreading viruses.
Projected Timelines for Vaccine Development and Trials
Experts are working on projected timelines for the development and trials of new vaccines, which are essential for planning and response strategies in future outbreaks. These timelines help in setting realistic expectations and preparing for the logistical aspects of vaccine distribution and administration.
In summary, the global response to infectious diseases now heavily relies on the swift development of vaccines facilitated by modern technologies like mRNA, with government and health organizations playing a central role in this process. The projected timelines for vaccine trials and development are critical for strategic planning and effective response to outbreaks.
Conclusion
Throughout this article, we have delved deep into the intersection of public health concerns, specifically the bird flu outbreak, and their consequential impact on the stock market, with a spotlight on Moderna and Pfizer. These discussions not only highlighted the critical importance of mRNA vaccines in combatting pandemic threats but also underscored the significant market movements triggered by emerging public health crises. It is evident that the advancements in vaccine technology, particularly mRNA technology, and the proactive roles played by biotech companies like Moderna, are integral to addressing such threats, reinforcing the relationship between public health developments and financial market dynamics.
Moreover, the global response to the avian flu, underscored by collaborative efforts between governments, health organizations, and biotech firms, illuminates the broader significance of these developments. Beyond the market reactions, this collaborative response emphasizes the critical importance of swift vaccine development and distribution in mitigating the impacts of infectious diseases. As the world continues to navigate the challenges of public health crises, the contributions of these entities remain indispensable, showcasing a future where public health safety and economic stability are increasingly interlinked, with continued innovation and strategic partnerships being key to navigating these complex landscapes.